Abstract and Introduction
Abstract
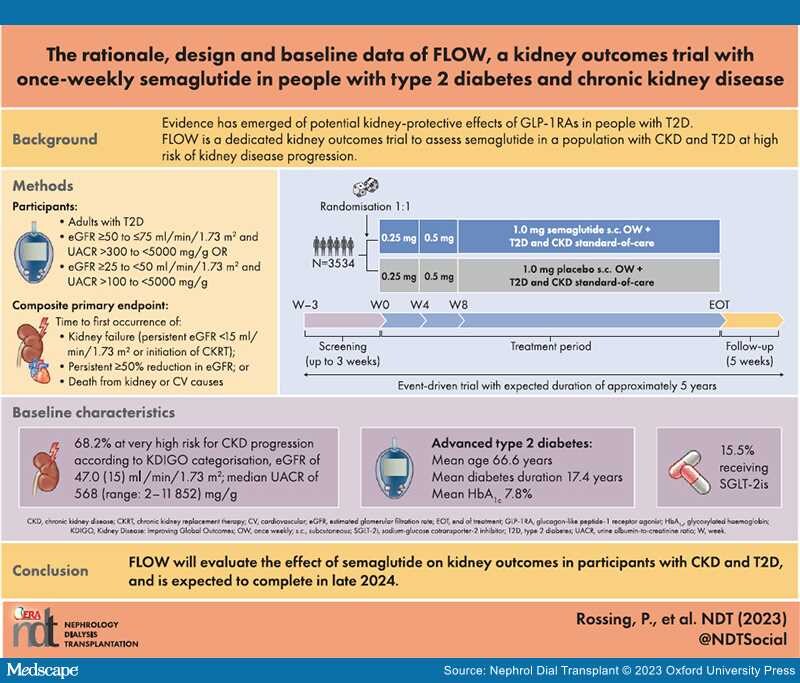
Background: Chronic kidney disease (CKD) is a common complication of type 2 diabetes (T2D). Glucagon-like peptide-1 receptor agonists (GLP-1RAs) improve glycaemic control and lower body weight in people with T2D, and some reduce the risk of cardiovascular (CV) events in those with high CV risk. GLP-1RAs might also have kidney-protective effects. We report the design and baseline data for FLOW (NCT03819153), a trial investigating the effects of semaglutide, a once-weekly (OW) GLP-1RA, on kidney outcomes in participants with CKD and T2D.
Methods: FLOW is a randomised, double-blind, parallel-group, multinational, phase 3b trial. Participants with T2D, estimated glomerular filtration rate (eGFR) ≥50−≤75 ml/min/1.73 m2 and urine albumin:creatinine ratio (UACR) >300−<5000 mg/g or eGFR ≥25−<50 ml/min/1.73 m2 and UACR >100−<5000 mg/g were randomised 1:1 to OW semaglutide 1.0 mg or matched placebo, with renin–angiotensin–aldosterone system blockade (unless not tolerated/contraindicated). The composite primary endpoint is time to first kidney failure (persistent eGFR <15 ml/min/1.73 m2 or initiation of chronic kidney replacement therapy), persistent ≥50% reduction in eGFR or death from kidney or CV causes.
Results: Enrolled participants (N = 3534) had a baseline mean age of 66.6 years [standard deviation (SD) 9.0], haemoglobin A1c of 7.8% (SD 1.3), diabetes duration of 17.4 years (SD 9.3), eGFR of 47.0 ml/min/1.73 m2 (SD 15.2) and median UACR of 568 mg/g (range 2−11 852). According to Kidney Disease: Improving Global Outcomes guidelines categorisation, 68.2% were at very high risk for CKD progression.
Conclusion: FLOW will evaluate the effect of semaglutide on kidney outcomes in participants with CKD and T2D, and is expected to be completed in late 2024.
Graphical Abstract
Introduction
Chronic kidney disease (CKD) is associated with increased morbidity and mortality, including an increased risk of both kidney failure and cardiovascular (CV) events.[1] CKD is a common complication of type 2 diabetes (T2D), affecting ≈40% of those with T2D, and the risk increases with diabetes duration.[1] The presence of CKD causes average healthcare costs to increase by almost 50% compared with costs for those with diabetes alone.[2]
Clinical practice guidelines recommend multifactorial interventions to reduce the risk of CKD developing or progressing in people with T2D.[3] Recommendations include optimising blood glucose levels towards an individualised target, lowering blood pressure, moderating dietary protein intake and weight loss. Guidelines on CKD and diabetes management, such as the 2022 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for patients with CKD and the 2022 American Diabetes Association (ADA) standards-of-care (SoC) guidelines for patients with T2D recommend treatment with renin–angiotensin–aldosterone system (RAAS) blocking agents and sodium–glucose co-transporter 2 inhibitors (SGLT2is) in most patients.[3,4] Both guidelines also recommend a non-steroidal mineralocorticoid receptor antagonist (finerenone) for patients with CKD at increased risk for CV events but unable to use SGLT2is.[4,5] The 2022 KDIGO guidelines recommend long-acting glucagon-like peptide-1 receptor agonists (GLP-1RAs) with proven CV benefits as the preferred second-line treatment for glucose lowering and CV event risk reduction in individuals either with CV disease (CVD) or at high risk of CVD who are not achieving individualised glycaemic targets despite the use of metformin and an SGLT2i or who are unable to use those medications.[3] For patients with confirmed atherosclerotic CVD or at high risk of CV events, the 2022 ADA SoC guidelines recommend either a GLP-1RA or an SGLT2i with proven CV benefits.[4]
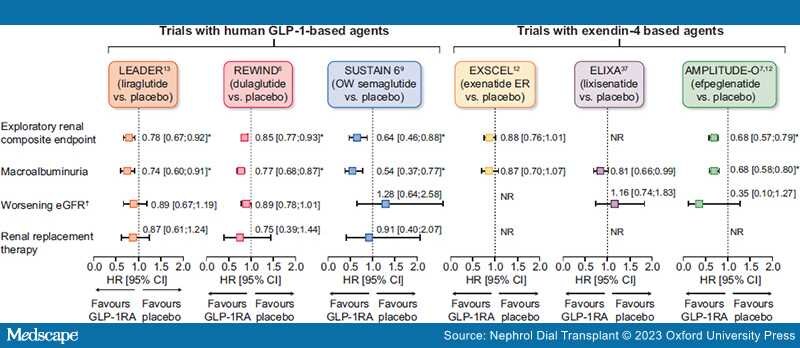
GLP-1RAs are an effective treatment for glycaemic control and weight reduction in people with T2D and have a good safety and tolerability profile (generally transient gastrointestinal adverse events being most common), including in those with CKD.[3,4] Some GLP-1RAs [albiglutide, efpeglenatide, dulaglutide, liraglutide and once-weekly (OW) semaglutide] reduce the risk of major adverse CV events (MACE) in people with T2D with established CVD or at high risk of CVD.[6–10] Meta-analyses of CV outcomes trials (CVOTs), which included >40 000 participants, suggest potential kidney-protective effects of GLP-1RAs, with some (liraglutide, dulaglutide, semaglutide and efpeglenatide) associated with reductions in secondary composite kidney outcomes (macroalbuminuria, substantial loss of kidney function, kidney failure and death due to kidney disease; Figure 1).[11,12] However, these benefits were driven by a lower risk of persistent macroalbuminuria.[6,10,13]
Figure 1.
Results of exploratory kidney analyses from GLP-1RA CV outcomes trials. *P < .05. †Defined as doubling of serum creatinine in LEADER, SUSTAIN 6 and ELIXA and sustained eGFR decline of ≥30% in REWIND. Direct comparisons not possible due to differing study designs. Secondary kidney composite endpoints were new-onset persistent macroalbuminuria/very high albuminuria, persistent doubling of the serum creatinine or end-stage kidney disease in LEADER; first occurrence of new macroalbuminuria/very high albuminuria (UACR >33.9 mg/mmol), a sustained decline in eGFR of ≥30% or chronic KRT in REWIND; new or worsening nephropathy (defined as persistent macroalbuminuria/very high albuminuria, persistent doubling of serum creatinine and a creatinine clearance of <45 ml/min/1.73 m2 or the need for continuous KRT) in SUSTAIN 6; a 40% decline in eGFR, KRT, kidney death or incident macroalbuminuria/very high albuminuria in EXSCEL; and incident macroalbuminuria (UACR >33.9 mg/mmol) plus an increase in UACR of ≥30% from baseline, sustained decrease in eGFR of ≥40% for ≥30 days, renal replacement therapy for ≥90 days and sustained eGFR <15 ml/min/1.73 m2 for ≥30 days in AMPLITUDE-O.6,7,10,13,38,43 exenatide ER, exenatide extended release; KRT, kidney replacement therapy; NR, not reported.
Despite improvements in kidney outcomes with available pharmacotherapies, the risk of kidney failure in people with CKD and T2D remains high.[14,15] Given the promising preliminary findings with GLP-1RAs, further exploration of this drug class as agents to improve kidney outcomes in people with CKD and T2D is a priority. FLOW (NCT03819153) is a dedicated kidney outcomes trial designed to determine the kidney-protective effects of semaglutide in participants with CKD and T2D, as well as assessing the effect on CVD mortality. Here we describe the study design and report the baseline characteristics of the FLOW population.
Nephrol Dial Transplant. 2023;38(9):2041-2051. © 2023 Oxford University Press